Background: Despite advances in cure rates for pediatric acute lymphoblastic leukemia (ALL), infections remain a significant cause of treatment-related morbidity and mortality. Patients with a central venous catheter (CVC) who develop fever while severely neutropenic (absolute neutrophil count (ANC) < 500) are known to be at high risk for serious infections such as blood stream infections (BSIs). However, on Children's Oncology Group (COG) ALL protocols, the majority of a patient's therapy course is in the maintenance phase, during which patients are typically not severely neutropenic. While the BSI rate in all pediatric oncology patients presenting with non-neutropenic fever has been reported to be around 5 to 10% (Allaway et al Pediatr Blood Cancer 2019), minimal data are available regarding BSI and other infections in pediatric ALL patients with non-neutropenic fever.
Objective: The objective of this study was to describe the infectious outcomes of children with ALL who developed non-neutropenic fever.
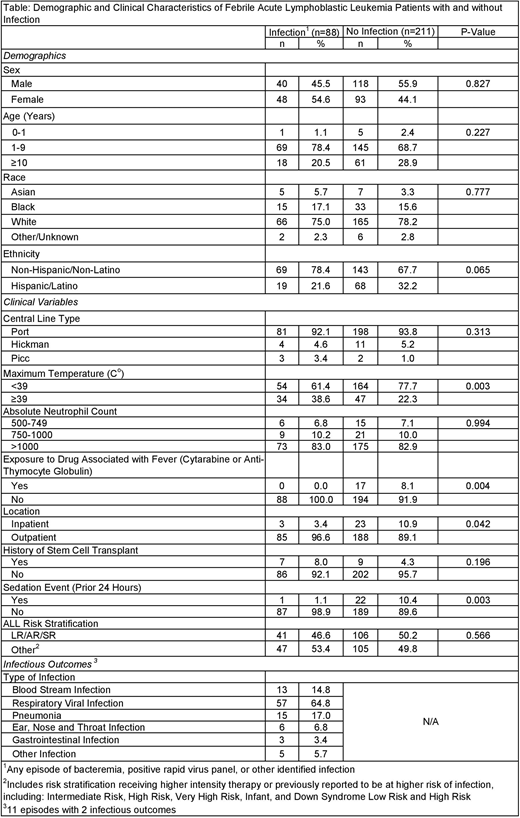
Methods: A single institution retrospective chart review collected data on all non-neutropenic pediatric ALL patients (age 0-25 years) with a CVC who were evaluated for fever (temperature ≥ 38ºC) and had a blood culture drawn at the time of presentation from April 2019 through June 2020. Febrile events occurring within 7 days of a prior febrile episode, during administration of empiric or treatment antibiotics, and in patients within 30 days after stem cell transplant (SCT) were excluded. The primary outcome was documented infection from the day of fever evaluation including BSI, positive respiratory viral panel (RVP) result, or other clinically identified infection. The following demographic and clinical variables were abstracted to evaluate risk factors for infection: age, sex, race, ethnicity, CVC type, maximum presenting temperature, ANC, exposure to chemotherapy agents known to be associated with fever (cytarabine or anti-thymocyte globulin), patient location at presentation, history of SCT, sedation event within the prior 24 hours, and COG ALL protocol risk stratification. Institutional review board approval was obtained. Descriptive and inferential statistics, including chi-square or Fisher's exact test as appropriate, were calculated. Analyses were performed using SAS Enterprise Guide v7.1.
Results: Data were collected on 299 non-neutropenic febrile episodes experienced by 150 patients. Eighty-eight (29.4%) episodes (61 patients) had at least one identified infection. Thirteen (14.8%) infections were a BSI; the overall BSI rate was 4.3%. Seven BSIs were gram-positive organisms and the remainder were gram-negative organisms. The other infections included 57 (64.8%) respiratory viral infections, 15 (17.0%) pneumonias, 6 (6.8%) ear, nose and throat infections, 3 (3.4%) gastrointestinal infections, and 5 (5.7%) other infections. There was more than one identified infection in 11 episodes (12.5% of infectious episodes) with respiratory viral infection and pneumonia as the most common co-infections (n=6). Presenting temperature ≥ 39ºC was associated with having an identifiable infection (p=0.003). Documented infections occurred less frequently in patients with an exposure to a drug associated with fever (p=0.004), who were inpatient at presentation (p=0.042), or who had a sedation in the prior 24 hours (p=0.003).
Conclusion: In this single institution cohort of children with ALL, 29.4% of non-neutropenic febrile episodes were due to a documented infection. The BSI rate of 4.3% is similar to other published reports of pediatric oncology patients with a CVC who developed non-neutropenic fever. Not surprisingly, the most common type of infection was respiratory viral infection. Notably, infections occurred more frequently in patients with a presenting temperature ≥ 39ºC while having had a sedation event within the past 24 hours or having received a chemotherapy agent associated with development of fever were less likely to be associated with an infection. These results provide data that may be useful in determining need for empiric antimicrobial therapy in pediatric ALL patients with non-neutropenic fever based on height of fever and recent treatment exposures. Further evaluation of these initial results in larger, multi-institutional studies are ongoing in order to guide future approaches to treatment of children with ALL who develop non-neutropenic fever.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.